Axona may nutritionally support cognition*1
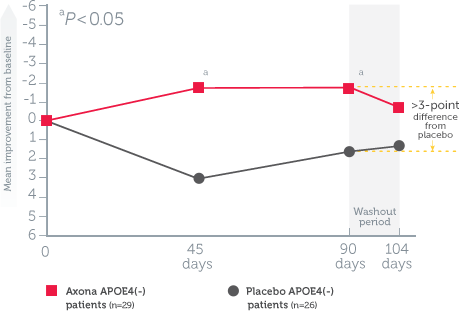
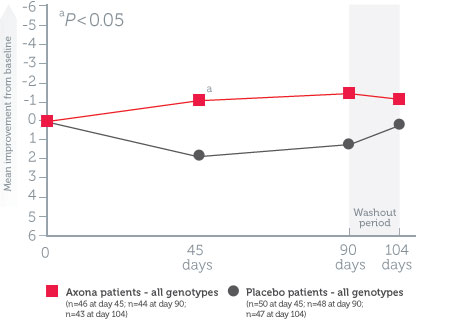
Axona improved Alzheimer’s Disease Assessment Scale–Cognitive subscale (ADAS–Cog) scores in APOE4(-) patients vs. placebo*1
- Approximately 80% of trial patients took Axona concurrently with one or more approved medications for Alzheimer’s disease1
- Axona may be used concurrently with acetylcholinesterase inhibitors and N-methyl-D-aspartic acid receptor antagonists1
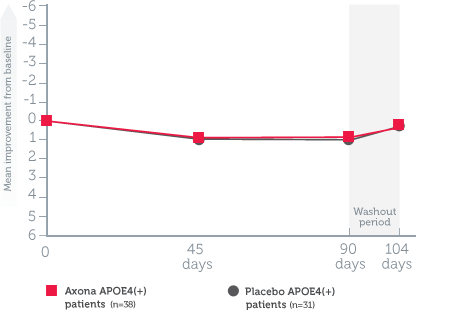
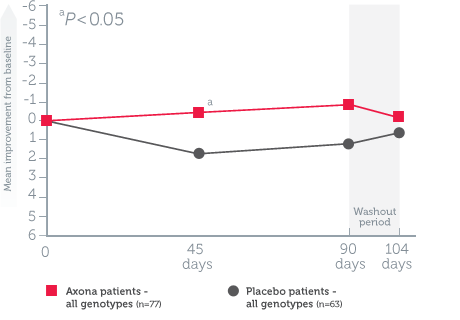
Mean change in ADAS–Cog scores from baseline in all randomized patients1
Study design1: The effect of daily Axona on memory and cognition was evaluated in a randomized, double-blind, placebo-controlled, 90-day, phase IIb, multicenter trial in 152 patients with probable mild to moderate Alzheimer’s disease. Patients were stratified by APOE4 status, and Axona was evaluated in several subpopulations. The primary endpoint was a significant improvement on ADAS–Cog at day 90. A preplanned secondary endpoint investigated whether efficacy was influenced by APOE4 status.
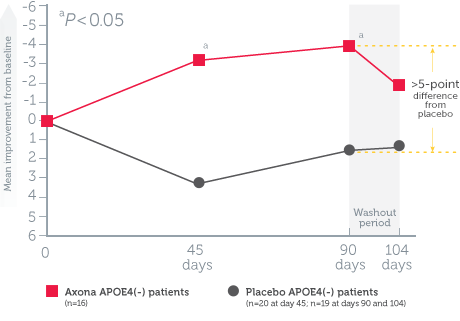
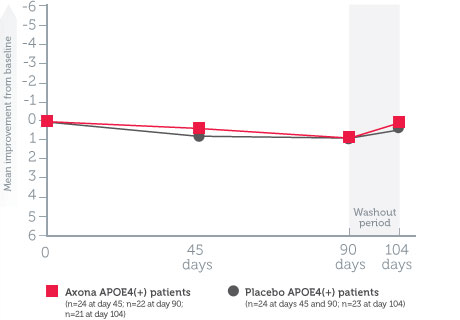
The effectiveness of Axona improved with dosage adherence*1
- Patients who consumed at least 80% of the total Axona dose over 90 days experienced the greatest improvement (5-point improvement vs. placebo)
- Patients began to decline when they stopped taking Axona during the 2-week washout period
Mean change in ADAS–Cog scores from baseline in dosage-adherent patients1
Study design1: The effect of daily Axona on memory and cognition was evaluated in a randomized, double-blind, placebo-controlled, 90-day, phase IIb, multicenter trial in 152 patients with probable mild to moderate Alzheimer’s disease. Patients were stratified by APOE4 status, and Axona was evaluated in several subpopulations. The primary endpoint was a significant improvement on ADAS–Cog at day 90. A preplanned secondary endpoint investigated whether efficacy was influenced by APOE4 status.
Cognitive performance correlated with the plasma concentration of ketone bodies1
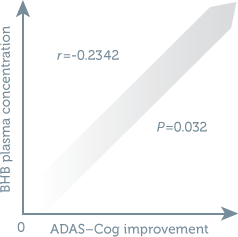
- Axona produced significantly elevated levels of the ketone β-hydroxybutyrate (BHB) at all postdose time points1
- Higher plasma concentrations of BHB ketone correlated with lower ADAS–Cog scores, irrespective of genotype1