Axona safety
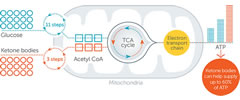
Axona increases ketone body concentrations to safe but effective levels*1
- The plasma concentration of β-hydroxybutyrate (BHB) ketone following Axona administration was mild, similar to that seen in the early phases of a low-carbohydrate diet
- When tested at twice the recommended dose, Axona had no significant effect on:
- - Total cholesterol
- - Very low-density lipoprotein (LDL) cholesterol
- - LDL cholesterol
- - High-density lipoprotein cholesterol
Plasma concentration of BHB ketone under various conditions1
| Intervention | Average BHB levels (mM) |
|---|---|
| › 12-hour fast |
0.08-0.1 |
| › Axona (20 g; 2 hours postdose) |
0.36 |
| › Low-carbohydrate diet |
0.4-0.65 |
| › Ketogenic diet |
0.3-1.6 |
| › Starvation (5-6 weeks) |
4-8 |
| › Diabetic ketoacidosis |
9-10 |
Adverse events were generally mild1,2
- Diarrhea was the most frequently reported adverse event; however, the incidence was reduced when Axona was mixed with a meal replacement drink
- Gas and nausea were other commonly reported adverse events
- Discontinuation rates due to any gastrointestinal-related event also declined once the Axona mixer was switched
- To reduce the chance of gastrointestinal adverse events, Axona should be titrated gradually over 7 days, before patients begin to take one 40 g packet per day
Order free Patient Starter Kits
Axona may be used concurrently with Alzheimer’s drug treatments1
- Approximately 80% of trial participants took Axona concurrently with one or more approved medications for Alzheimer’s disease
- Axona may be taken alone or with Alzheimer's disease therapies, including acetylcholinesterase inhibitors and N-methyl-D-aspartic acid receptor antagonists
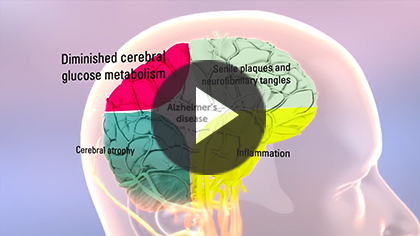
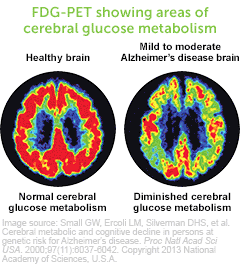
Learn more about how Axona fits into mild to moderate Alzheimer’s disease management
Axona contains safe ingredients2
- Axona is manufactured, labeled, and distributed in accordance with strict Food and Drug Administration (FDA) guidelines
- All ingredients are Generally Recognized As Safe or FDA-approved food additives
Safety information2
- Axona is a prescription medical food intended for the clinical dietary management of the metabolic processes associated with mild to moderate Alzheimer's disease
- Axona should be used with caution in patients who are at risk for ketoacidosis, for example, patients with a history of alcohol abuse and poorly controlled diabetics; or those who have a history of inflammation of the gastrointestinal system, metabolic syndrome, and/or renal dysfunction. Axona contains caseinate and whey (dairy), and lecithin (soy). Contains: milk and soy